FluQuadri® Vaccine
MRP ₹ 9,275
Composition: Inactivated quadrivalent influenza Vaccine
Dosage form and strength: Suspension for injection, Prefilled single-dose storage syringe, 0.5ml
Route of administration: For Intramuscular use only
Single pack contains 5 doses
DESCRIPTION
FluQuadri® suspension for injection is clear and slightly opalescent in color. FluQuadri® (Quadrivalent Influenza Vaccine) for intramuscular injection is an inactivated influenza vaccine. FluQuadri® is an inactivated quadrivalent influenza vaccine indicated for the prevention of influenza disease caused by influenza types A and B viruses contained in the vaccine.
COMPOSITION
The single-dose, pre-filled syringe (0.5 mL) manufactured and formulated without thimerosal or any other preservative. The amounts of HA and other ingredients per dose of vaccine are listed below.
FluQuadri® Ingredients
| Ingredient | FluQuadri® 0.5 mL Dose |
|---|---|
| Active Substance: Split influenza virus, inactivated strains*: | 60 mcg HA total |
| A/Sydney/5/2021 (H1N1)pdm09 - like strain | 15 mcg HA |
| A/Darwin/9/2021 (H3N2) - like strain | 15 mcg HA |
| B/Austria/1359417/2021- (B/Victoria lineage)- like strain | 15 mcg HA |
| B/Phuket/3073/2013 –(B/Yamagata lineage)- like strain | 15 mcg HA |
| Inactive ingredients | |
| Sodium chloride | 6.6 g/L |
| Sodium phosphate dibasic anhydrous | 3.830 g/L |
| Sodium phosphate monobasic anhydrous | 0.410 g/L |
| Formaldehyde | ≤100 mcg |
| Octylphenol ethoxylate (Triton® X-100) | ≤250 mcg |
*Will vary year to year based on the strains selected as per WHO recommendations for each NH and SH Influenza season.
CLINICAL INFORMATION
FluQuadri® is approved for use in persons 6 months of age & older.
Dose and Schedule for FluQuadri®
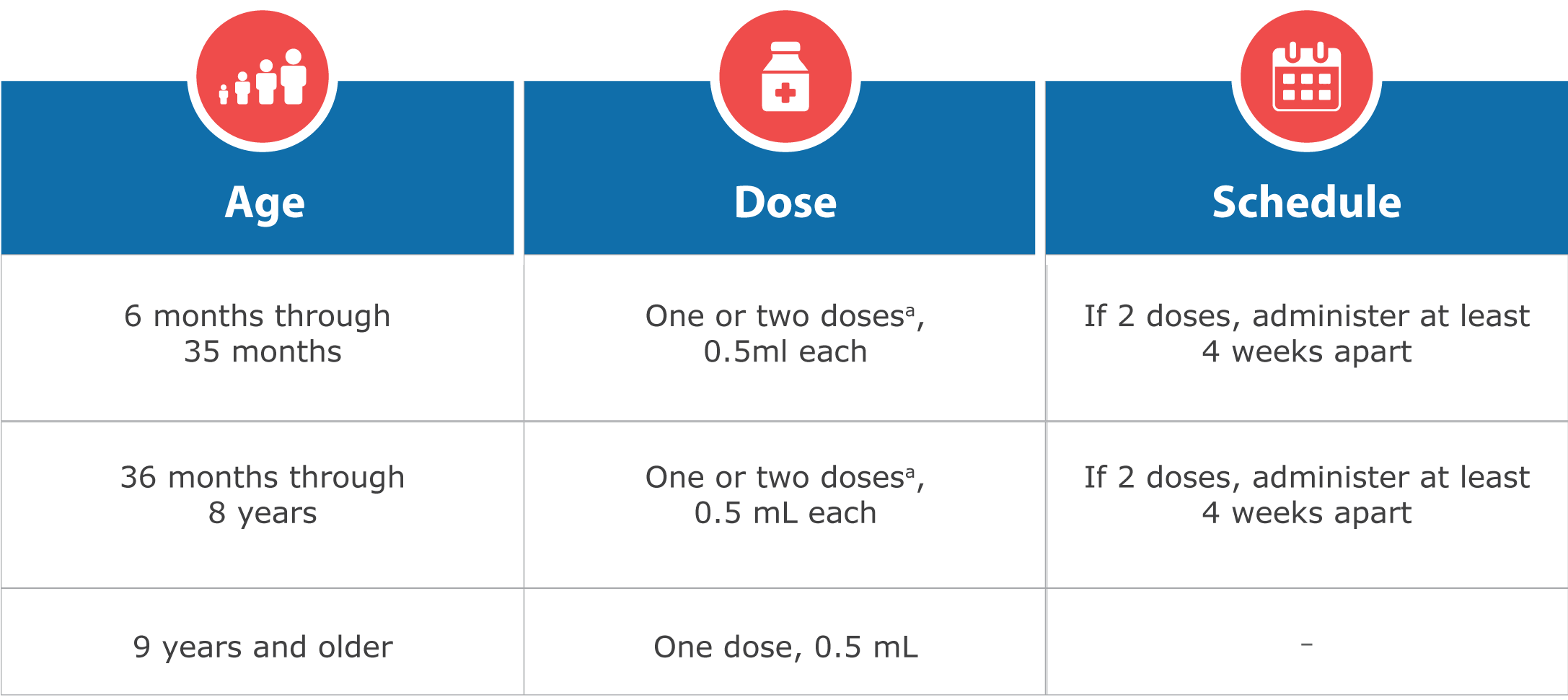
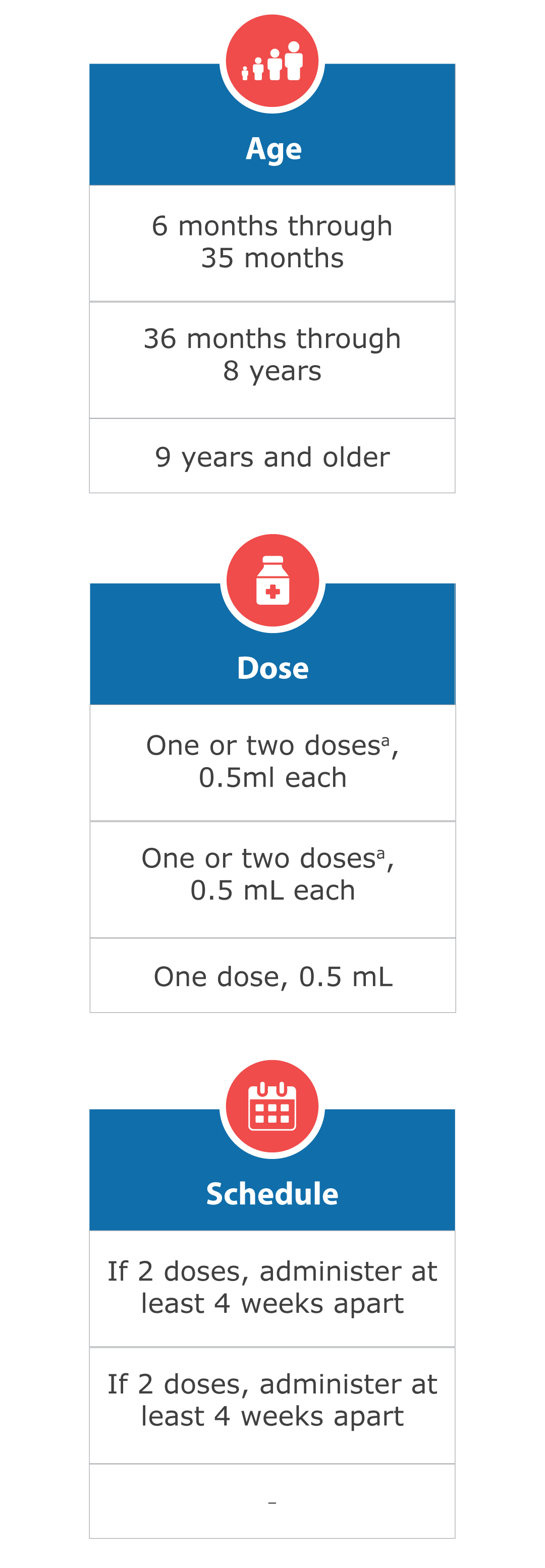
a 1 or 2 doses depends on vaccination history as per Advisory Committee on Immunization Practices annual recommendations on prevention and control of influenza with vaccines.
HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Single-dose, prefilled syringe (clear plunger rod), without needle, 0.5 mL, package of 5 (not made with natural rubber latex)
Storage and Handling
Store all FluQuadri® presentations refrigerated at 2° to 8°C (35° to 46°F). DO NOT FREEZE. Discard if vaccine has been frozen. Do not use after the expiration date shown on the label. The shelf life of FluQuadri® is 12 months.