Hexaxim® Vaccine
MRP ₹ 4,095
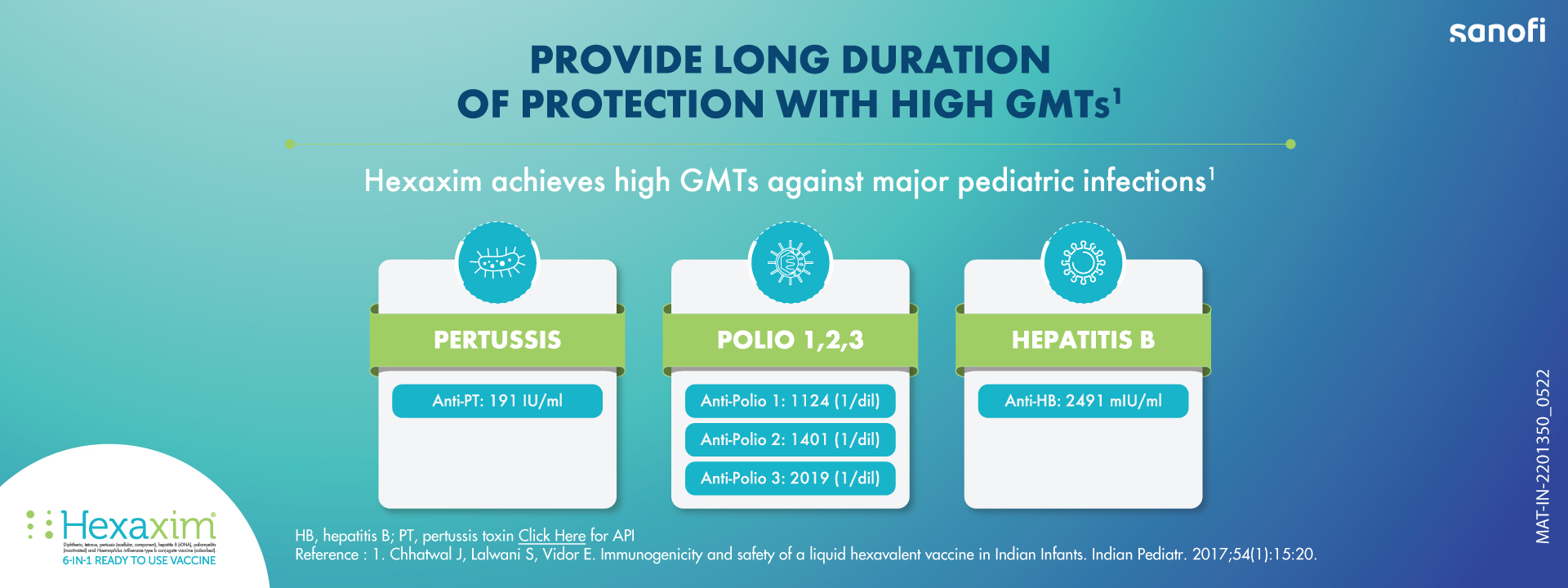
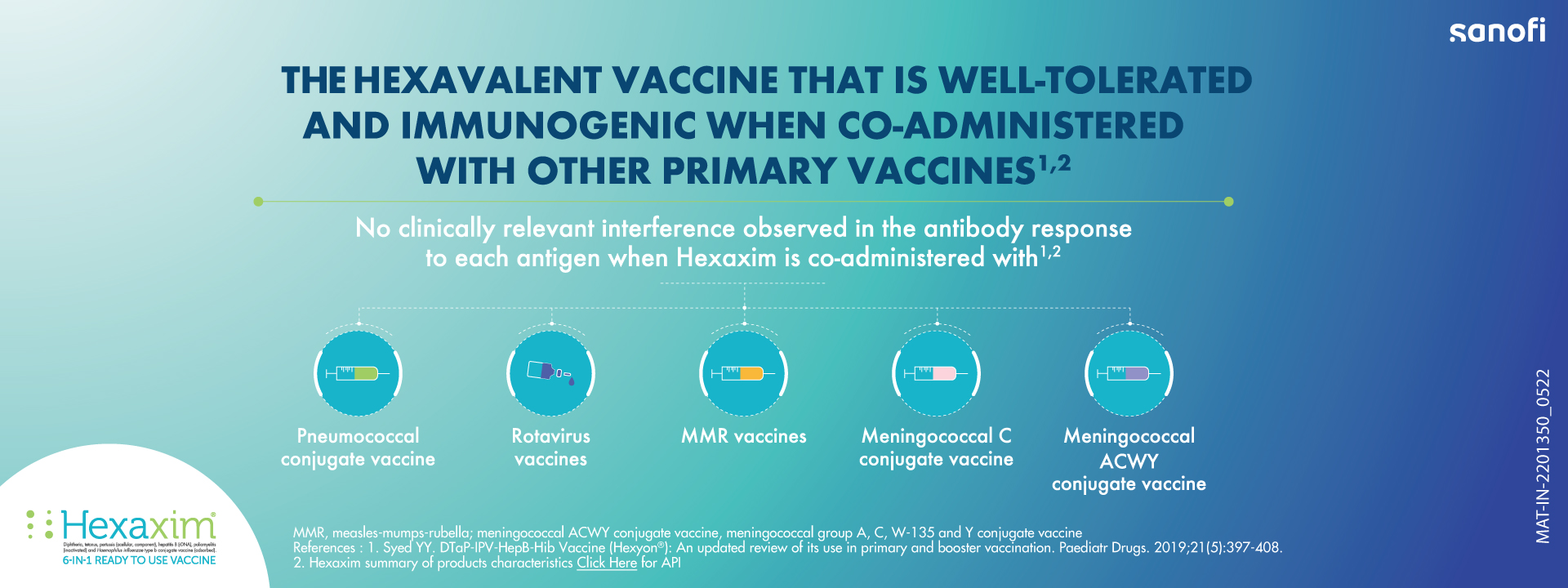
Hexaxim® vaccine is used as a protection against infectious diseases. It is intended for primary vaccination of infants and toddlers from 6 weeks of age and booster vaccination up to 24 months of age against diphtheria, pertussis, tetanus, hepatitis B, poliomyelitis and invasive diseases caused by Haemophilus influenzae b (Hib). The vaccine works by producing antibodies to act against bacteria and viruses that cause these infections.
DESCRIPTION
Hexaxim® is thiomersal-free, whitish cloudy, ready-to-use suspension vaccine. It is a new hexavalent combination vaccine comprising of diphtheria and tetanus toxoids, inactivated poliovirus vaccine (IPV; type 1,2, and 3), two acellular pertussis antigens (PT and PHA), H. influenzae type b vaccine (Hib), consisting of H. influenzae type b polysaccharide (polyribosilribitol phosphate) conjugated to tetanus toxoid (PRP-T) and hepatitis B vaccine (Hep B) consisting of recombinant hepatitis B virus surface antigen (HBsAg) produced in the Hansenula polymorpha yeast. Reconstitution of any component of vaccine is not required and thus, offers easy administration with low risk of medication error.
Hexaxim® is available as a single dose, pre-filled syringe of fully liquid 0.5mL suspension. It is administered as intramuscular injection in the antero-lateral aspect of the upper thigh in infants and toddlers and the deltoid muscle in older children.
CLINICAL INFORMATION1
Hexaxim® is approved to be used for primary vaccination of infants from 6 weeks of age and booster vaccination up to 24 months of age.
Dosing frequency of Hexaxim®
STORAGE AND HANDLING
Store at 2°C to 8°C (35°F to 46°F). DO NOT FREEZE. Keep the vaccine in the outer carton in order to protect from light. Do not use after expiration date shown on the label.
| Vaccination type | Dosing interval |
|---|---|
| Primary vaccination | Three injections administered at an interval of one or two months (at least 4 weeks apart) |
| Booster vaccination | Single dose to be administered at least 6 months after the last dose of primary vaccination up to 24 months of age. |
References: 1. Hexaxim® vaccine [Prescribing information]. Sanofi Pasteur Inc.