Tetraxim® Vaccine
MRP ₹ 2,188
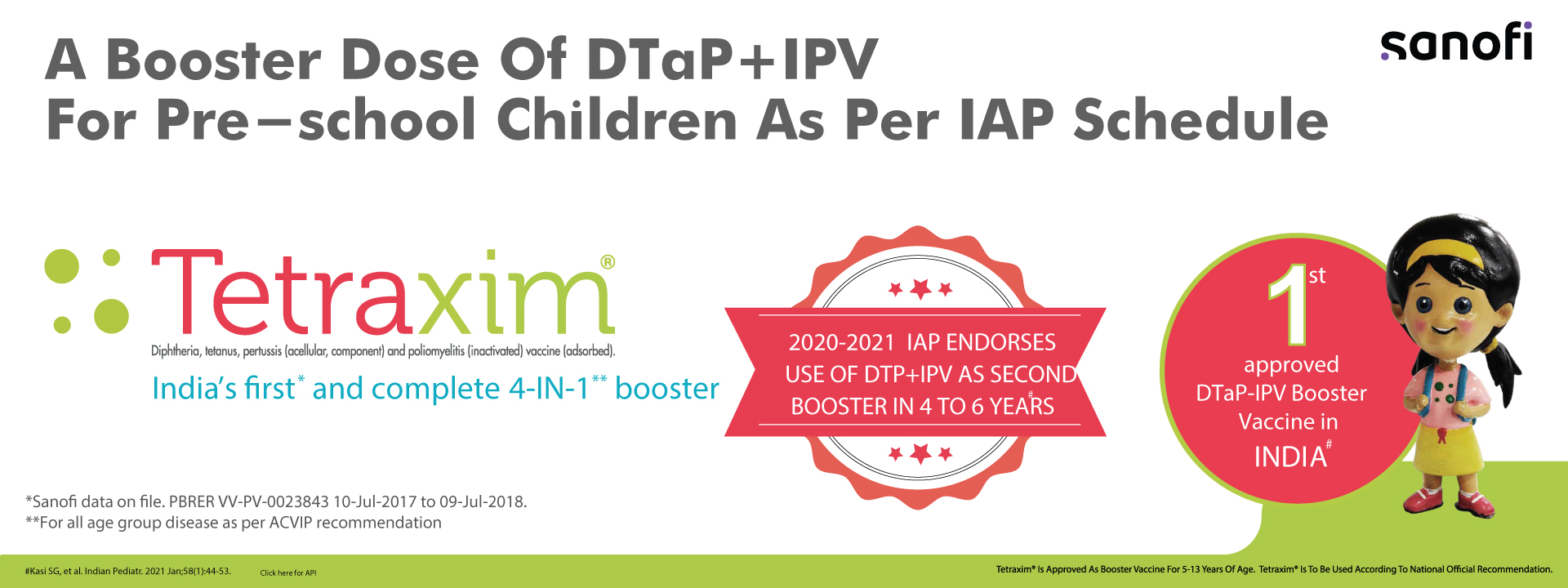
Tetraxim® is a tetravalent adjuvanted combined vaccine which is indicated in the joint prevention of diphtheria, tetanus, pertussis
and poliomyelitis:
• For primary vaccination in infants from the age of 2 months,
• For booster vaccination, one year after primary vaccination during the second
year of life,
• For booster vaccination between 5 and 13 years of age, according to official
recommendations.
This type of infection does not cause illness but helps to stimulate body immune system to produce antibodies to protect against any future infections.
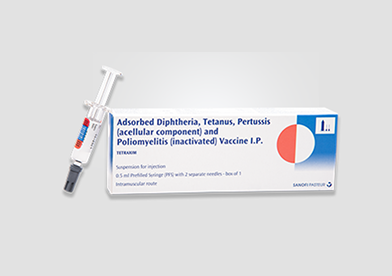
DESCRIPTION
Tetraxim® vaccine is a whitish-turbid sterile suspension available in the form of pre-filled syringe (with or without needle) containing a dose of 0.5 mL. Tetraxim® is a combination of aluminium hydroxide as an adjuvant and four drug substances – purified diphtheria toxoid (D), purified tetanus toxoid (T), Bordetella pertussis antigens: pertussis toxoid and filamentous haemagglutinin (FHA) and an inactivated poliovirus type 1,2, and 3 (IPV).
For syringes without the attached needle, the separate syringe must be attached firmly to the syringe, by rotating it in a one-quarter turn. Shake before injection until a homogeneous cloudy, whitish suspension is obtained.
Tetraxim® is a single dose vaccine to be administered by the intramuscular route. The vaccine should be preferably administered in the deltoid area in children between 5 and 13 years of age. Tetraxim® vaccine complies with the European Pharmacopoeia (Ph.Eur.) and the WHO recommendations.1
CLINICAL INFORMATION2
Tetraxim® is approved to be used for booster vaccination between 5 and 13 years of age.
Dosing frequency of Tetraxim®
| Vaccination type | Dosing interval |
|---|---|
| Booster vaccination | Injection administered between 5 and 13 years of age. |
References: 1. National institute of Pharmacy and Nutrition. Public Assessment Report, Tetaxim https://mri.cts-mrp.eu/Human/Downloads/HU_H_0406_001_PARSummary.pdf. Accessed on March 14, 2022. 2. Tetraxim® vaccine [Summary of Product Characteristics]. Sanofi Pasteur Inc.